In previous discussions in this series, we have shown how scientists are reluctant to let go of paradigms. That's not necessarily a bad thing. It forces new thoeries to provide a better explanation of how things work and cautiously advances our knowledge and understanding of the universe.
One of the basic paradigms in modern physics has to do with atomic decay. This refers to the action whereby unstable atoms shed particles in their nucleus to make them stable. We know much about this decay and about which kinds of atoms shed which types of particles. We even know how long it takes for a certain mass of unstable atoms to shed these particles. This is known as the rate of decay and it is considered to be very stable and predictable. But we don't know precisely when this decay will happen. It's always random.
A chunk of radioactive cesium-137, for example, may decay at a steady rate overall, but individual atoms within the chunk will decay in an unpredictable, random pattern. Thus the timing of the random ticks of a Geiger counter placed near the cesium might be used to generate random numbers.
 Random number generators are used all the time in scientific experiments and in computer science to make cryptograms. A typical atomic (quantum) random number generator measures the bursts of particles being thrown off by radioactive material. The detector measures the time between the bursts and uses this measure in a mathematical formula to arrive at a random number.
Random number generators are used all the time in scientific experiments and in computer science to make cryptograms. A typical atomic (quantum) random number generator measures the bursts of particles being thrown off by radioactive material. The detector measures the time between the bursts and uses this measure in a mathematical formula to arrive at a random number.
The rate of decay for various unstable atoms has been documented for decades and is assumed to be unchanging. But that assumption was challenged in an unexpected way by a group of researchers from Purdue University who were, at the time, more interested in random numbers than nuclear decay.
Ephraim Fischbach, a physics professor at Purdue, was looking into the rate of radioactive decay of several isotopes as a possible source of a quantum random number generator. He began his investigation by reviewing the recently published decay rates for various unstable elements and noticed something odd -- they did not agree with each other!
Reviewing the data collected at Brookhaven National Laboratory and the Federal Physical and Technical Institute in Germany, he noticed that the reported decay rate of silicon-32 and radium-226 showed a seasonal variation, being slightly faster in winter than in summer.
These findings strengthened Fischbach's suspicion that the strange swings in decay rates were caused by the Sun. The swings seemed to be in synch with the Earth's elliptical orbit, with the decay rates oscillating as the Earth came closer to the Sun and then moving away.
Was this fluctuation real, or was it merely a glitch in the equipment used to measure the decay? Could the equipment be influenced by the change of seasons, with the accompanying changes in temperature and humidity?
"Everyone thought it must be due to experimental mistakes, because we're all brought up to believe that decay rates are constant." -- Peter Sturrock, professor emeritus of applied physics
While scientists were pondering that data, another anomaly was found.
On Dec 13, 2006, a solar flare sent a stream of plasma particles and radiation toward the Earth. Purdue nuclear engineer, Jere Jenkins, was busy measuring the decay rate of manganese-54, a short-lived isotope used in medical diagnostics. He noticed that the decay rate dropped slightly about 36 hours before the flare began and returned to normal when the flare was finished.
Jenkins first thought was that this phenomenon could be useful in predicting solar flares which could help prevent damage to satellites and electric grids, as well as save the lives of astronauts in space. But the mechanism involved in this phenomenon was bizarre.
The decay rate aberrations that Jenkins noticed occurred during the middle of the night in Indiana -- meaning that if it was something produced by the sun, it would have had to travel all the way through the Earth to reach Jenkins' detectors. What kind of energy could do that?
 Jenkins and his associate, Fischbach, concluded that the only particle capable of penetrating the earth and coming from the Sun was a neutrino. Neutrinos are almost weightless particles famous for flying at almost the speed of light through the physical world -- humans, rocks, oceans or planets -- with virtually no interaction with anything.
Jenkins and his associate, Fischbach, concluded that the only particle capable of penetrating the earth and coming from the Sun was a neutrino. Neutrinos are almost weightless particles famous for flying at almost the speed of light through the physical world -- humans, rocks, oceans or planets -- with virtually no interaction with anything.
Critics didn't want to believe this phenomenon was valid. In a series of papers published in Astroparticle Physics, Nuclear Instruments and Methods in Physics Research and Space Science Reviews, Jenkins, Fischbach and their colleagues showed that the observed variations in decay rates were highly unlikely to have come from environmental influences on the detection systems. They were real.
The suspicion that these anomalies were possibly related to solar neutrinos was made stronger when Peter Sturrock (Professor Emeritus at Stanford), suggested that the Purdue scientists look for other recurring patterns in decay rates. As an expert of the inner workings of the sun, Sturrock had a hunch that solar neutrinos might held the key to this mystery. As he expected, the Purdue researchers noticed the decay rates vary repeatedly every 33 days -- a period of time that matches the rotational period of the core of the sun.
The usual changes on the surface of the Sun are in 28 day cycles. The core rotates faster than the surface. The solar core now appears to be the source of the decay anomalies and the strange neutrinos. But how? Neutrinos aren't supposed to work like that.
| Here come the neutrinos!
A neutrino (Italian, meaning "small neutral one") is an elementary particle, meaning that it is not made up of smaller particles, such as quarks etc. In this regard it is like an electron and photon and unlike protons and neutrons (which are composed of quarks etc.).
Neutrinos have almost no mass and no electrical charge. This allows them to behave like "ghost particles" where they usually travel close to the speed of light and are able to pass through ordinary matter almost unaffected. In physics, they are denoted by the Greek letter v (nu).
Neutrinos are everywhere. They evolved in huge quantities during the theoretical "Big Bang" that created the universe. Some believe that neutrinos are the "dark matter" that alludes scientists because, even though their mass in extremely small, there are so many of them that their cumulative effect could just be the force that holds everything together [3].
Right now, neutrinos are passing through your body. Although some come from cosmic space (every second, in the region of the Earth, about 65 billion solar neutrinos pass through every square centimeter perpendicular to the direction of the sun), most come from our own Sun. They are a by-product of the fusion process that also gives us heat and light (see the chart below).
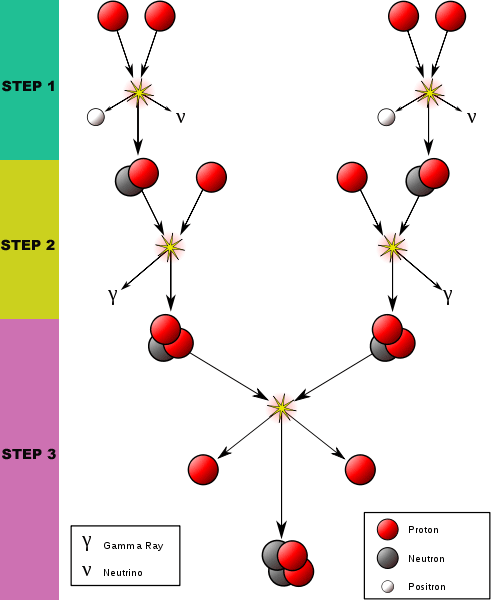
[Above:] The interior of the Sun is hot enough to strip the single electron from an atom of Hydrogen, which is the simplest and most numerous atom in the universe. It consists of one central proton and one orbiting electron. In Step 1, two protons (i.e. two hydrogen atoms without their electrons) join together releasing their excess energy as an electron (-) and a positron (+) and (0.42 MeV) excess energy. A neutrino is also released in this reaction -- the same solar neutrinos that are the subject of interest in our story.
 The electron and positron particles immediately annihilate each other because of their opposite charges, releasing more (1.03 MeV) energy, and two gamma ray photons.
The electron and positron particles immediately annihilate each other because of their opposite charges, releasing more (1.03 MeV) energy, and two gamma ray photons.
Very quickly, one of the two newly joined protons changes to become a neutron, causing it to become Deuterium -- a form of hydrogen with one proton and one neutron.
In Step 2, the Deuterium nucleus joins with another free proton and becomes Helium-3. Helium is the next larger atom after hydrogen. It usually has only two electrons and two protons but, in this form, it has two protons plus a neutron in its nucleus. This process releases excess energy (5.49 MeV) and a gamma ray photon.
In Step 3 the two atoms of Helium-3 join together to become Helium-4. This form of Helium has 2 protons and two neutrons in its core. Obviously, the recipe calls for less protons than is required, so two protons are released to begin their subsequent lives in more similar reactions inside the Sun. This is the most common reaction in the Sun (86%).
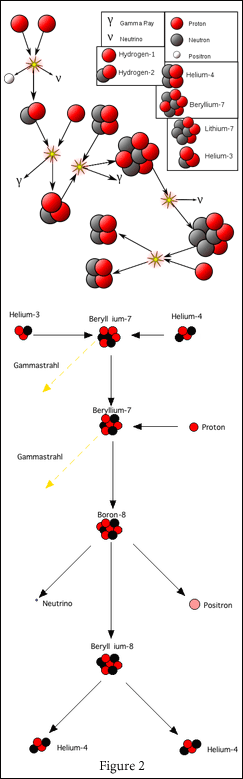
From the creation of Helium-4, there are two other possible ways that these atoms can react.
One [Figure 2, top] involves the joining of Helium-4 with Helium-3, producing Beryllium-7 (4 protons and 3 neutrons), where one of the protons changes to a neutron (creating Lithium-7), releasing a neutrino before splitting again to form two atoms of Helium-4 and a proton. This reaction releases 12.86 MeV of energy and is the second most common fusion reaction in the Sun (14%).
The other reaction [Figure 2, bottom] is like the previously described one, except that the Beryllium-7 captures a proton and becomes Boron-8, which then splits to shed a positron and a neutrino, becoming Beryllium-8 before splitting yet again to become two Helium-4 atoms. This releases 14.06 MeV of energy. This is a very rare reaction, happening only 0.11% of the time.
Confused?
This is the kind of stuff that gives headaches to ordinary people, like me. But it is good to know that the production of neutrinos is a part of the Sun's energy cycle and that these particles bathe us, inside and out, and perhaps are the most numerous particles in the whole universe. It's also why we should be very concerned if they are suddenly behaving in some way that is transforming matter, since that is what you and I are made from.
As we will see, some scientists balk at the idea that neutrinos can be responsible for changes in the decay rate. But what they propose is something even more radical -- a completely new particle!
|
More particle weirdness
If we insist on holding on to our current understanding about neutrinos, there is a possibility that this unexpected effect is brought about by a previously unknown particle emitted by the sun.
"What we're suggesting is that something that doesn't really interact with anything is changing something that can't be changed... It's an effect that no one yet understands. Theorists are starting to say, 'What's going on?' But that's what the evidence points to. It's a challenge for the physicists and a challenge for the solar people too.
[If the mystery particle is not a neutrino], It would have to be something we don't know about, an unknown particle that is also emitted by the sun and has this effect, and that would be even more remarkable." -- Peter Sturrock
|
Researchers try to reassure worried scientists thatRadiometric Dating is still reliable
Atoms of radioactive isotopes are unstable and decay over time by shooting off particles at a fixed rate, transmuting the material into a more stable substance. For instance, half the mass of carbon-14, an unstable isotope of carbon, will decay into nitrogen-14 over a period of 5,730 years. The stable regularity of this decay allows archaeologists to determine the age of extremely old organic materials -- such as remains of Paleolithic campfires -- with a high degree of precision. The decay of uranium-238, which has a half-life of nearly 4.5 billion years, enabled geologists to determine the age of the Earth.
 Many scientists, including Marie and Pierre Curie, Ernest Rutherford and George de Hevesy, have attempted to influence the rate of radioactive decay in their laboratories by radically changing the pressure, temperature, magnetic field, acceleration, or radiation environment of the source. No experiment to date has detected any change in rates of decay from these kinds of influence. Many scientists, including Marie and Pierre Curie, Ernest Rutherford and George de Hevesy, have attempted to influence the rate of radioactive decay in their laboratories by radically changing the pressure, temperature, magnetic field, acceleration, or radiation environment of the source. No experiment to date has detected any change in rates of decay from these kinds of influence.
With so much science hanging in the balance, researchers from NIST and Purdue tested this decay anomaly by comparing radioactive gold-198 in two shapes, spheres and thin foils, with the same mass and activity. Gold-198 releases neutrinos as it decays. The team reasoned that if neutrinos are affecting the decay rate, the atoms in the spheres should decay more slowly than the atoms in the foil because the neutrinos emitted by the atoms in the spheres would have a greater chance of interacting with their neighboring atoms.
The maximum neutrino flux in the sample in their experiments was several times greater than the flux of neutrinos from the sun. The researchers followed the gamma-ray emission rate of each source for several weeks and found no difference between the decay rate of the spheres and the corresponding foils. They quickly denounced the whole phenomenon as an unexplained quirk.
The problem with this experiment is that the neutrinos causing the observed changes in atomic decay are not those produced by the sample itself (in this case the Gold-198), but rather those allegedly coming from the Sun (which are presumed to be different in some way). So we should expect no change in the two samples, regardless of their shape. They would both be affected by this new (or old) external particle, as are all other observed elements with this same anomaly.
Once again, paradigms stubbornly persist and those with an interest in maintaining the staus quo will go to any length to assure the public that things as we know it have not changed.
|
Back to the expanding Earth theory...
In Part 1 of this series, we explored the Expanding Earth Theory. Perhaps the main objection voiced by skeptics is how any solar radiation, such as plasma, could penetrate the Earth's core and actually transform matter. I think the phenomenon described in this article reveal that such an occurrence is not only possible, but is likely already a reality.
Plamsa contains neutrinos, as well as other types of radiation. Since they can pass through the planet, they can certainly have an effect on the core. As we have seen with the atomic decay anomalies, this effect can transmute matter -- causing atoms to become different elements. This anomaly appears to be something that occurs in cycles related to solar activity. Since we are in one of the most predicted active solar cycles we may well expect some epic changes here on Earth.
These same changes may also be happening in our bodies, which contain numerous unstable atoms. Are we in a state of transformation that will not only effect our planet -- but our species as well?
What do you think? We'd like to hear your comments.
 Viewzone || Comments
Viewzone || Comments
Notes:
[1] R.M. Lindstrom, E. Fischbach, J.B. Buncher, G.L. Greene, J.H. Jenkins, D.E. Krause, J.J. Mattes and A. Yue. Study of the dependence of 198Au half-life on source geometry. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. doi:10.1016/j.nima.2010.06.270
[2] http://science.slashdot.org/story/10/09/25/0254219/Scientists-Confirm-Nuclear-Decay-Rate-Constancy
[3] The Particle Odessey (2002) by Frank Close, Michael Martin, Christine Sutton, ISBN 0 19 850486 1
 Viewzone || Comments
Viewzone || Comments
COMMENTS:


 Random number generators are used all the time in scientific experiments and in computer science to make cryptograms. A typical atomic (quantum) random number generator measures the bursts of particles being thrown off by radioactive material. The detector measures the time between the bursts and uses this measure in a mathematical formula to arrive at a random number.
Random number generators are used all the time in scientific experiments and in computer science to make cryptograms. A typical atomic (quantum) random number generator measures the bursts of particles being thrown off by radioactive material. The detector measures the time between the bursts and uses this measure in a mathematical formula to arrive at a random number. Jenkins and his associate, Fischbach, concluded that the only particle capable of penetrating the earth and coming from the Sun was a neutrino. Neutrinos are almost weightless particles famous for flying at almost the speed of light through the physical world -- humans, rocks, oceans or planets -- with virtually no interaction with anything.
Jenkins and his associate, Fischbach, concluded that the only particle capable of penetrating the earth and coming from the Sun was a neutrino. Neutrinos are almost weightless particles famous for flying at almost the speed of light through the physical world -- humans, rocks, oceans or planets -- with virtually no interaction with anything.
 The electron and positron particles immediately annihilate each other because of their opposite charges, releasing more (1.03 MeV) energy, and two gamma ray photons.
The electron and positron particles immediately annihilate each other because of their opposite charges, releasing more (1.03 MeV) energy, and two gamma ray photons.  Many scientists, including Marie and Pierre Curie, Ernest Rutherford and George de Hevesy, have attempted to influence the rate of radioactive decay in their laboratories by radically changing the pressure, temperature, magnetic field, acceleration, or radiation environment of the source. No experiment to date has detected any change in rates of decay from these kinds of influence.
Many scientists, including Marie and Pierre Curie, Ernest Rutherford and George de Hevesy, have attempted to influence the rate of radioactive decay in their laboratories by radically changing the pressure, temperature, magnetic field, acceleration, or radiation environment of the source. No experiment to date has detected any change in rates of decay from these kinds of influence.